Liberating Hydrogen Using Aluminum-Water Reaction
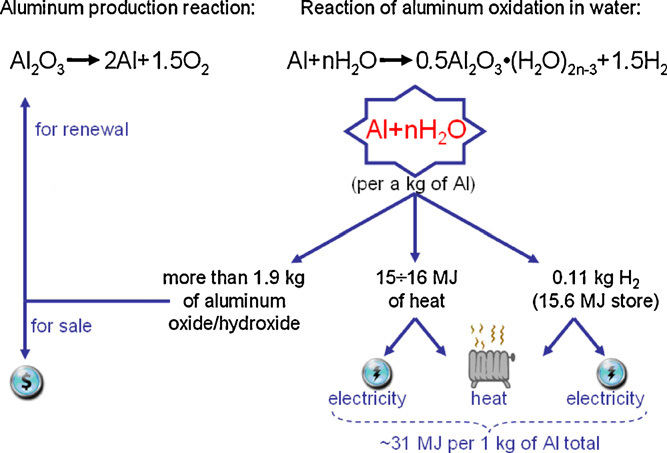
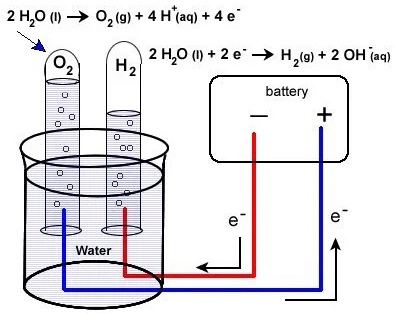
The production of hydrogen through the reaction of aluminum with water has recently proved to be efficient. During the process, aluminum reacts with water to produce aluminum hydroxide and hydrogen gas. The reaction begins with heat or a catalyst, producing hydrogen gas. This simple process boosts efficiency and scalability, ideal for localized, on-demand hydrogen generation.
Reaction Process: When there is a reaction between aluminum and water, hydrogen gas and aluminum hydroxide are formed. The reaction rate improves with a catalyst or by heating aluminum for greater reactivity.
High Yield: It is a highly-yielding reaction, where a large volume of hydrogen is produced from a relatively small quantity of aluminum.
On-demand Hydrogen: A significant benefit of this process is that it produces hydrogen as required, reducing transportation and storage costs.